Corrosion
Heavy-duty anchor Guidebook – Part 5
In the next article of our guide on the topic of heavy-duty anchoring, we will show that there are other external influences besides loads that play a major role in the choice of fastening elements, including corrosion. Corrosion involves a chemical-physical reaction of a metallic material with its environment. This reaction can lead to changes in the properties of the material, which impair the function of the metal, the component or the entire construction.
The following types of corrosion are important for metallic connections in heavy-duty anchoring:
The following types of corrosion are important for metallic connections in heavy-duty anchoring:
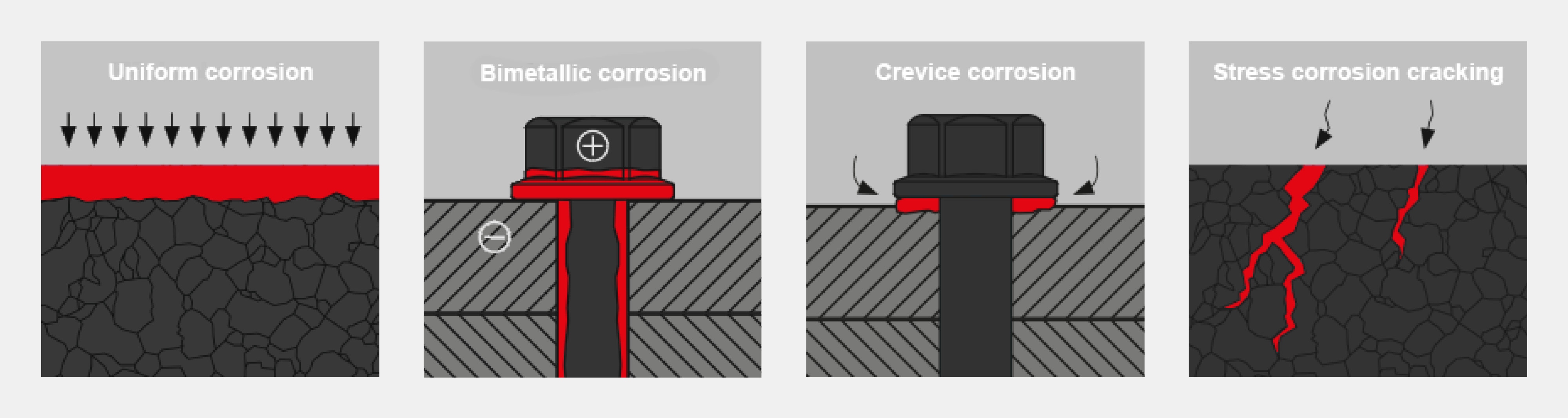
Uniform corrosion
A characteristic feature of uniform corrosion is the consistent abrasion of the surface through the formation of anodic and cathodic sections. Uniform corrosion primarily affects the surface properties and leads to optical impairments. With regard to the load-carrying capacity of the connection, this type of corrosion is rather uncritical.Bimetallic corrosion
Bimetallic corrosion occurs when two or more metallic materials with different voltage potentials come into contact. If a suitable electrolyte is present, the corrosion process occurs due to the different potentials of the two materials. This so-called galvanic corrosion can be prevented by selecting the appropriate combination of materials.Crevice corrosion
Crevice corrosion leads to a chemical decomposition of the material in narrow, unsealed crevices that are not adequately ventilated. The reactions that take place can also make the corrosion medium even more aggressive. Due to the lack of oxygen supply, no protective passive coating forms in the area of the crevice. The lack of this protective layer means that even alleged stainless steels can be attacked in the area of crevices.
Stress corrosion cracking
Stress corrosion cracking is very critical. The material is damaged by a combination of mechanical and chemical stress. Without the appearance of visible corrosion products, cracks in the material structure, embrittlement and, in the worst case, breakage can occur.Preventing corrosion damage
Corrosion protection measures begin with product design and the selection of suitable materials. A4 stainless steel is often used in anchoring technology. The steels are characterised by good corrosion resistance in moderately aggressive atmospheres. HCR stainless steels (e.g. 1.4529) should be used in particularly aggressive environments. These have the highest corrosion resistance in the range of stainless steels.If it is not possible to process corrosion-resistant materials, a number of coatings are available that improve corrosion protection: For metal anchors in the construction sector, galvanizing, hot-dip galvanizing or zinc alloys are generally used.
In general, the term "corrosion resistant" is used when suitable materials, such as for example stainless steels are used to avoid corrosion. If coating is applied to a steel surface for corrosion protection purposes, this is referred to as “corrosion-protected”. Finally, we would like to point out that the ETAs prescribe corrosion-protected anchors indoors and corrosion-resistant anchors outdoors.
For more detailed information, we recommend our corrosion guidebook and our corossion brochure.